Methane, A New Perspective
The Marsh Gas
Methane has been in our atmosphere since the dawn of Earth time.
Methane was considered to be one of the prebiotic gases of the atmosphere along with ammonia, nitrogen, hydrogen, CO2 and water.
It would have been involved with early metabolism in anaerobic microbes from the beginning of life on this planet, some 3.5 billion years ago.
Fair to say then, that it has been a natural gas in our atmosphere long before humans walked the Earth.
Its role in biology then is fairly simple;
Everything alive on Earth today will die.
Everything that is dead, will be decomposed by microbes (if not incinerated).
Everything that is decomposing will produce some methane
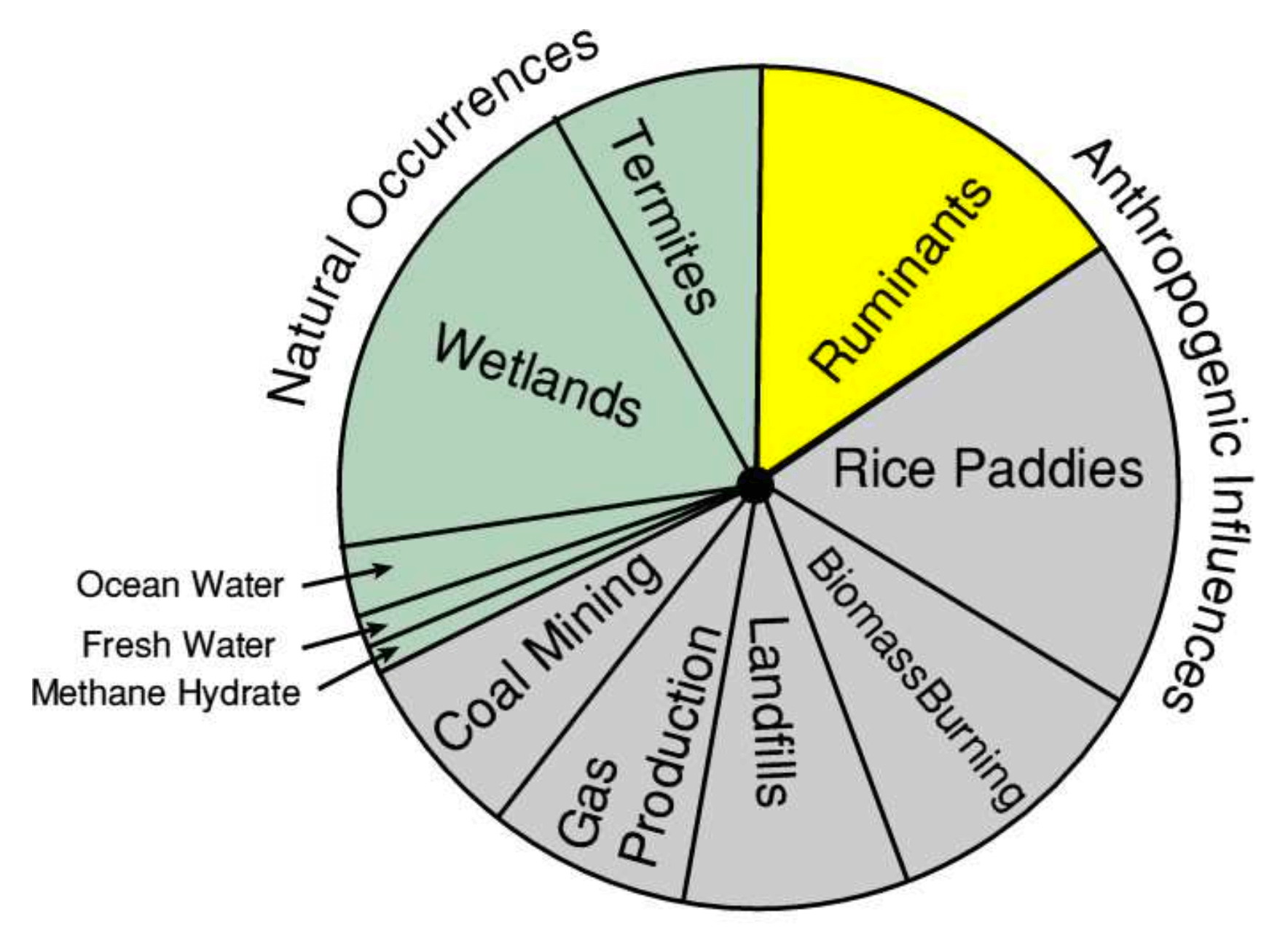
Apart from general decomposition, methane is also the product of digestion in termites and ruminants. Wetlands, rice paddies and landfill are simply specific examples of decompositional environments.
There are also abiotic sources of methane such as seepage from fossil fuel deposits, clathrates (methane trapped inside water or ice lattice) and in gas production.
On the other side, methane is oxidised in the atmosphere to carbon dioxide and water. A process that starts in the lower atmosphere but accelerates with altitude. Oxidation of methane is a major source of water in the stratosphere where water at roughly 4 parts per million (ppm) is double that of methane.
In addition microbes called methanotrophs breakdown methane throughout the biosphere.
Conclusion - Methane in our atmosphere is natural.
Methane Sources
.
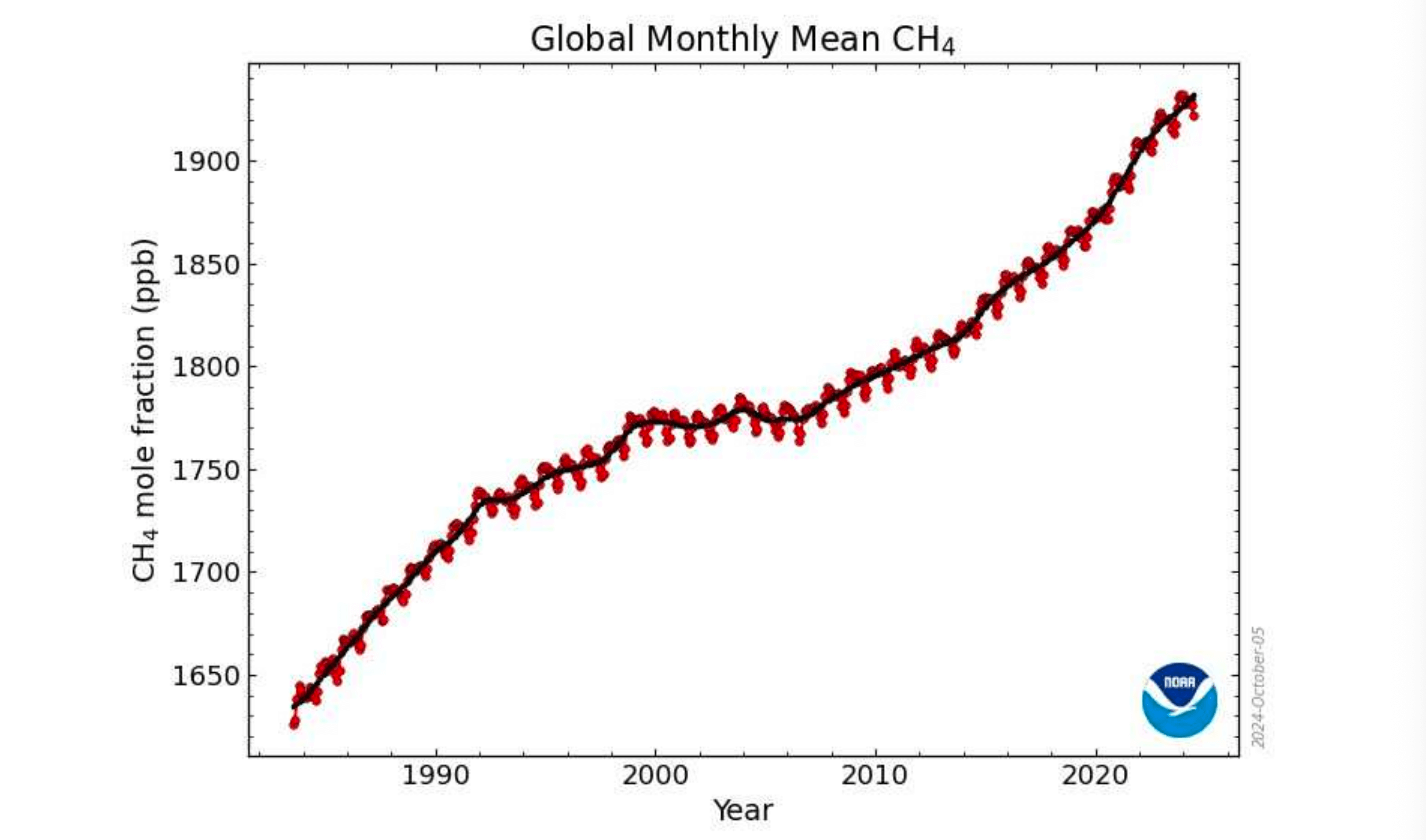
Concentration Of Methane In The Atmosphere
Since 1984 methane has risen from 1.64 ppm to 1.92 ppm. This rise has been attributed to the increase in Earths temperature over that time. Decomposition occurring at a faster rate in warm conditions,
(Rigby et al 2017 https://doi.org/10.1073/pnas.2411212121)
Concentration of Methane in Atmosphere.
Note: the pause in the rate of increase between 1998 and 2008 which coincided with a plateau in the global temperature record.
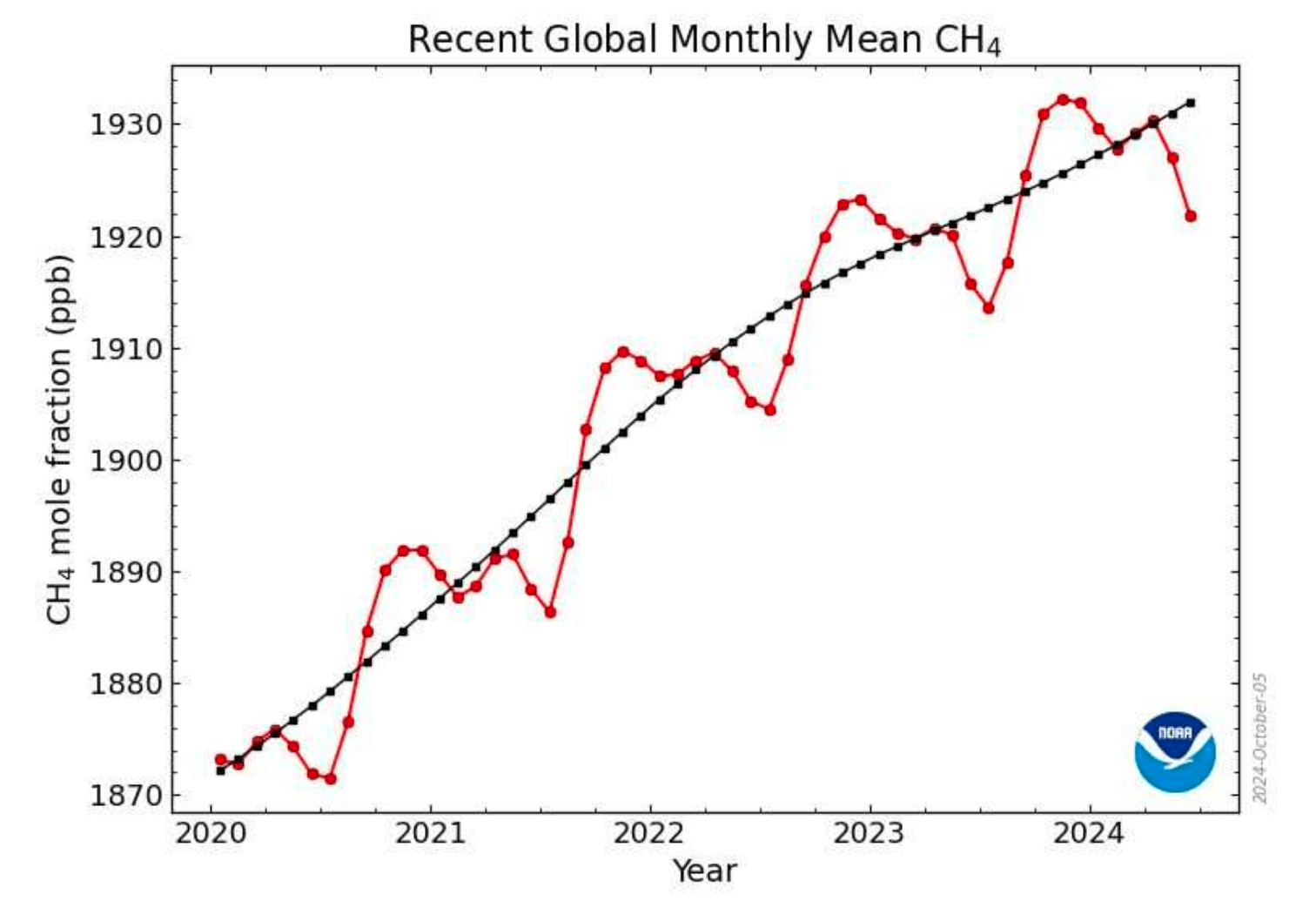
Recent Monthly Global Mean, Methane
.
Examination in more detail shows an annual variation in methane. The major rise in CH4 occurs during the rainy
season, from June to November. The minor methane peak occurs during the spring melt and early summer, typically from March -April in temperate and boreal regions (e.g., Canada, Russia, Northern Europe). However, from November to June with the exception of the minor peak, CH4 is decreasing.
In the period from June to December the increase is of the order of 0.2 ppm and the decline from December to June is about 0.1ppm.
The decline is important because it shows that during that period, oxidation of methane to CO2 and water occurs faster than methanes formation. Clearly the concentration of methane in the atmosphere is determined by two factors, the rate of formation of methane and its rate of destruction.
When formation > destruction - concentration increases.
When destruction > formation - concentration decreases
Now consider your bank balance, if your expenditure = your income then your balance stays the same, if money is flowing in faster than you withdraw it, you are getting richer but if money is flowing out faster than it is coming in then your balance decreases. The only thing that matters is whether the flow in is greater, the same, or smaller than the flow out (expenditure)
The concentration of CH4 in the atmosphere then is like your bank balance, it depends only on the inflow to the atmosphere and and outflow from it.
This is a steady state system
How long any cent of your wealth stays in the bank is utterly irrelevant.
How long any GHG molecule lasts in the atmosphere is also utterly irrelevant.
What matters is the inflow versus outflow - Steady state
Tyranny of numbers
The global warming effect from NZ livestock for the 100 year period 2000 to 2100 has been calculated by;
Professor David Frame (Canterbury), using IPCC methodology to be 0.0004°C.
Professor William van Wijngaarden (York, Canada) (Independent scientist) to be 0.0003 °C
These numbers are not measurable.
Put in perspective. A November day in Dunedin dawned with a temperature of 8°C, by mid afternoon it was 28°C. A 20°C.rise in one day. Where does 3 or 4 millionths of a degree, in a year, sit alongside that ?
Error Margins
For air temperature readings, errors are of the order of ± 0.2 to ± 0.5°C.
Therefore, the calculated increase from NZ livestock over 100 years, is about a thousand times smaller than the error margin of todays thermometers.
The increase in global temperature from NZ livestock can be safely rounded to zero
Got it - the effect of killing all NZ livestock would have no effect whatsoever on the measurable temperature of the world.
If the total warming from livestock over 100 years in NZ is not measurable, then;
• How do you measure a 30% reduction
• How do you measure the warming effect per farm, let alone reductions
If you cannot measure it then you cannot tax it
Furthermore, if we removed all ruminants from NZ the effect would be
• No meat (steaks, roasts, sausages, ham, burghers)
• No milk
• No Cheese
• No Yoghurt
• No leather
• No pet food
AND
• No effect whatsoever on methane emissions or global warming
AND
• lots of rotting grass producing as much methane as before
Rotten Science - The IPCC - GWP
The IPCC was set up with the mandate to show that humans caused global warming (now called climate change). because of their use of fossil fuels.
Human CO2 is calculated to be about 3-5 % of total annual CO2 released into the atmosphere from biological activity and volcanoes. About one quarter of that global CO2 is turned over every year, going into living organisms via photosynthesis and photo-plankton and out via respiration.
In order to blame humans for the increase in CO2 it was necessary to claim that what we put into the atmosphere stayed there. As a consequence of this need, a model was developed (Bern Model) that made out that some of our CO2 would stay in the atmosphere for a thousand years or more so what we put into the air from burning fossil fuels just added to that already in the atmosphere.
Almost every paper published (apart from the IPCC) gave the longevity of CO2 in the atmosphere as somewhere between 5 and 10 years. Furthermore one cannot distinguish between CO2 from human sources and CO2 from non human sources. Chemically CO2 is CO2 regardless of source. Recent research has shown that the increase in global CO2 has in fact come from the ocean. As the sea surface temperatures increase more CO2 is released. In fact, atmospheric CO2 is controlled by Henry’s Law, the temperature at the interface between air and water and in the ocean by Le Chateliers principle which controls the equilibrium between bicarbonate and dissolved CO2.
However, as a result of this emphasis, on how long a GreenHouse Gas (GHG) lasted in the atmosphere, the scientists aligned to the IPCC developed the Global Warming Potential as a way of comparing the effect of different GHGs.
This Global Warming Potential metric is not scientific by three measures;
The IPCC compares the GHGs by mass, but, a molecule of CO2 is 2.75 times heavier than a molecule of methane. Absorption of infrared radiation is a molecular property- not one of mass. This automatically overstates the role of methane by 2.75 times.
The IPCC looks at the increase in the absorption of infrared by an extra molecule. At low concentrations only a fraction of the available energy is absorbed, so a small increase in concentration has a significant effect. With CO2 almost all energy radiated is absorbed and transferred to the atmosphere so an increase in CO2 has little effect. By focusing on the increase in absorption and ignoring the total energy transferred, the IPCC greatly overstates the role of methane and nitrous oxide.
In calculating GWP the IPCC ignores the overlap in absorption by water. Water absorbs/re-radiates the same wavelengths of radiation as methane (and nitrous oxide) Imagine you are a photon of infrared trying to get from one goalpost to the other, ahead of you are 15000 water molecules and 1 methane. Who is going to give you the most trouble ?
As a result of this competition, water suppresses the absorbing power of methane by a factor of >18 times.
GWP is a political concept it has no scientific merit
“ENERGY TRANSFER:
Earth radiates infrared. GHGs absorb much of this radiated energy which is immediately transferred to other molecules (mainly N2 and O2 ) either by collision OR by the GHG radiating another photon to be absorbed by another GHG”
The IPCC - Methane
Methane is regarded as a short lived GHG, lasting only 10-12 years in the atmosphere. Despite this the IPCC applied the same methodology to methane with respect to longevity and came up with a GWP 100 of 28 or a GWP 20 of 85 (the superscript is the number of years involved).
Sara Mikaloff-Fletcher, a senior Scientist at NIWA told the Primary Production Select Committee, April 7th 2022, when discussing Climate Mitigation, “methane is a short lived greenhouse gas, but it has a very big impact on radiative forcing (heating) in the first 10 to 20 years. So it is the methane I emit today is going to impact the atmosphere, influence radiative forcing 85X more than an equivalent amount of CO2 that I emit today”
One has to question the scientific rationality of tying the effect of a GHG to how long it lives in the atmosphere. Including a longevity factor is to predetermine what the concentration will be in 20 or 100 years time. Given the uncertainties, that presumption is not scientifically based.
The effect of a GHG is determined by its concentration and its ability to absorb and radiate infrared in the atmosphere today.
As we have seen, that concentration is only determined by the inflow versus the outflow. What that concentration will be in 10 years or 100 years is no more than an ill-informed guess. Will oxidation increase or decrease, will rate of formation increase with continued warming or will the world undergo a natural cooling phase, in which case the formation of methane will reduce and oxidation will prevail and the concentration will drop.
How do they get that over 20 years the forcing of methane will be 85 times that of CO2 when the maximum forcing per molecule at present concentration is only 30 times (ignoring the fact that the warming from that is minuscule and the time taken to double methane is some 250 years) Methane only lasts 10 to 12 years by their own reckoning,so how is its effect so high 10 years after it has disappeared ?
In reality, we are dealing with a steady state system;
Every molecule of CH4 released from a cow today is matched by one CH4 being oxidised in the upper atmosphere today. The net effect is zero
The failure of IPCC calcuations is the failure to recognise the rate of oxidation of methane.
Temperature increases from methane are calculated on the assumption that the methane emissions from ruminants ADD to the global total CH4, they take no account that an equivalent amount of methane has been oxidised.
That is the CH4 from the cow is replacing CH4 that has been oxidised to CO2 in the atmosphere, not adding to it.
This is the guts of it - the IPCC does not understand the steady state system which every biochemist has understood for 70 years and every engineer has understood for millennia.
The concentration is determined by flow in vs flow out, not by how long an individual molecule lasts.
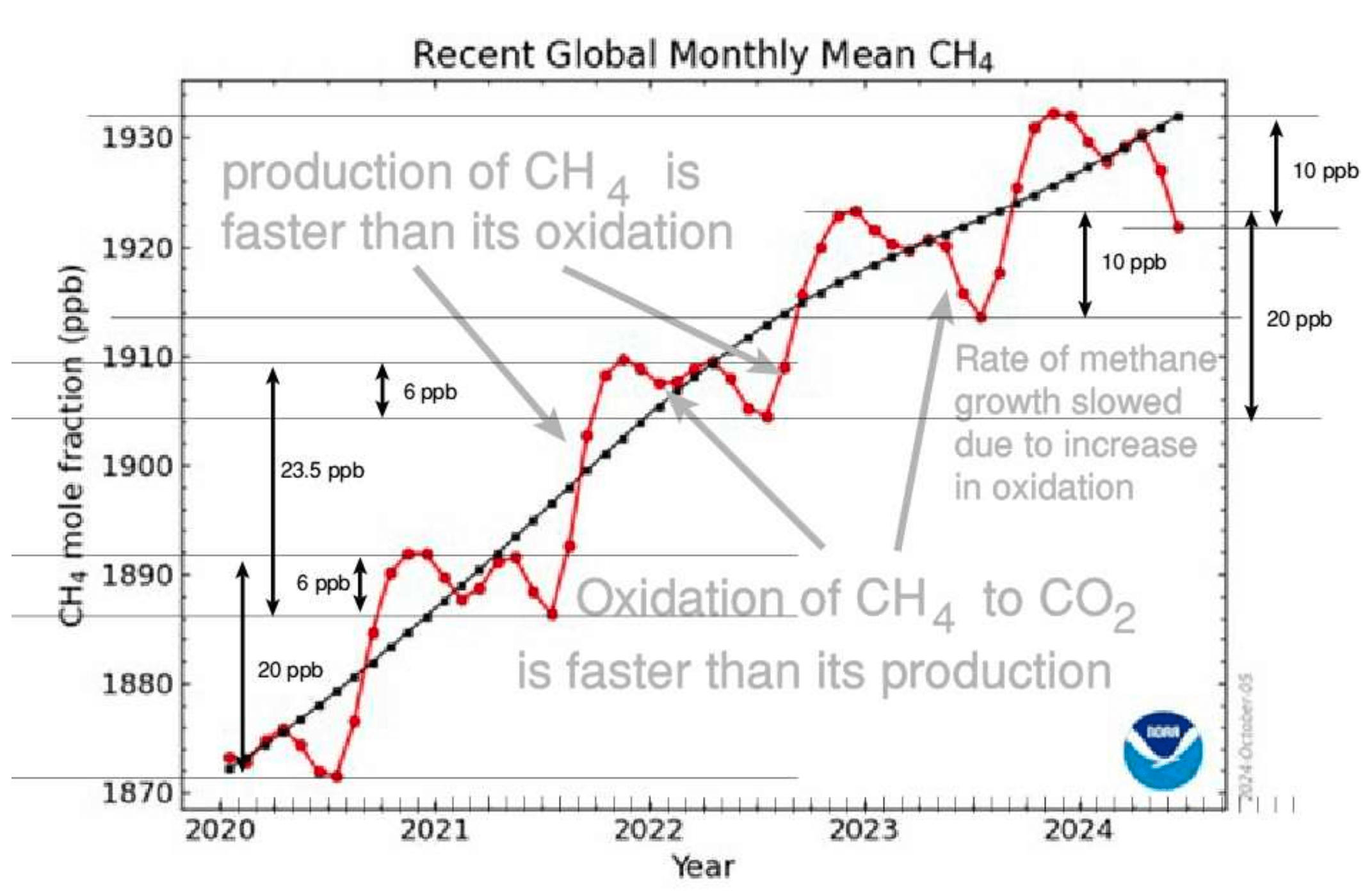
Examine the graph in
more detail.
The steep rises during the rainy season amount to an increase of 20 ppb as CH4 production exceeds oxidation. But during the November to June period oxidation is occurring at a faster rate than CH4 synthesis, so the level declines.
Note also the inflection in the slope of the graph in late 2022 as the rate of oxidation increased from 6 ppb to 10 ppb.
No net increase in CH4
Occurred in the period 1998 to 2008.
This is because all methane emissions world wide were matched one for one with oxidation of CH4 to CO2.
In this period, no methane released from the Earth had any warming effect whatsoever, but the Paris Accord still wants to destroy your herd for it.
Finally, Let’s Demolish Gwp
So, lets take their GWP 100 of methane being 28 times worse than CO2. Firstly divide it by 2.75 times to get both CO2 and CH4 on a per molecule basis.
Our 28 now becomes ~10
Since CH4 is suppressed 18 times by water then our GWP =10/18 = 0.55
With a couple of quick corrections their methane GWP changes from 28 times worse than CO2, to only producing half as much warming as CO2.
With thanks to Deborah Alexander and Emeritus Professor Geoff Duffy for their contributions.