Beginners Guide to Methane
Once upon a time Will-o’-the-wisps, the dancing ephemeral lights over swamps were things of wonder and source of much folk lore. We now understand that anaerobic (without oxygen) decomposition of organic matter produces a mixture of methane, phosphine and diphosphane. The latter ignite spontaneously on contact with air also igniting the methane giving rise to the ghostly lights often seen over swamps.
Methane then is a natural gas produced wherever decomposition of organic matter occurs. Decomposition requires water and warmth, so wet areas like wetlands, swamps, rice paddies stagnant ponds etc become natural sources of methane. Going back to the last decades of the 20th century it became popular to make bio-digesters where plant matter was fed into sealed vats and allowed to ferment, the gas (methane) given off was collected, pressurised and used as fuel for vehicle engines. In fact we had CNG (Compressed Natural Gas) used in place of petrol. CNG in those days was mainly sourced from the Taranaki gas field.
Four hundred and twenty million years ago the first plants evolved to have specialised roots systems, a trunk and a leafy top (Lycopodia) There were many varieties of these plants, some growing to 50+ metres. The problem was that when they died the bacteria and fungi at the time had no means of breaking down the lignans that comprised the bulk of the trunks and branches.
As a consequence, plants grew on top of dead plants until the pile of dead undecomposed matter was hundreds of metres thick. Gradually these piles of organic matter became buried by sediments and tectonic movement took them deeper. Over millions of years the heat and pressure turned these fossil seams into coal. However, higher temperatures of burial also caused more gas to form. Coal mine explosions result from accumulations of this coal gas which is a mixture of methane, carbon monoxide and hydrogen. Cities used to have gas reticulated throughout the city made by heating coal and driving out the gas.
Even without human intervention, gas from coal seams has always found its way to the surface. Oil forms similarly but from dead marine organisms accumulating on the seafloor and eventually covered by sediments. The deeper they were buried the hotter they became and over millions of years were converted to oils but if temperatures rose too high then gas was produced.
Methane production by animals.
All animals produce some methane in their flatus, it is a natural product of decomposition within the gut and is produced mainly in small intestine or caecum, however, ruminant animals developed a 4 chambered stomach to provide a chamber for microorganisms to break down the indigestible component of plants, cellulose. Cellulose comprises about 40 - 50% of dry plant matter. it is a long chain of glucose molecules but unlike starch, the bond between the glucose molecules cannot be broken by animal enzymes, that requires microbes.
Ruminants appeared some 50 million years ago so they predate humans by some 46 - 47 million years. Before humans ploughed up every bit of decent land on the planet, ruminants were a very successful animal and roamed every continent in their billions. Now some 90% of ruminants are farmed on a fraction of the land they once roamed.
Methane, whether seeping through the ground from coal seams, or as a product of decomposition either in swamps or in animal stomachs or intestines is natural, not anthropogenic. It has been occurring for at least 450 million years. Humans,on the other hand, have only been around for a few million years. Growing crops for food only dates back some 10,000 years.
Conclusion: Methane is a natural substance and has been a normal component of our atmosphere for billions of years
Why does decomposition of organic matter either in swamps or internal within animals produce methane.
Long chain carbohydrates like cellulose are broken down to small fatty acids like acetate and pyruvate. In this process the cells energy carriers all become converted to a reduced form that holds electrons and associated hydrogen ions. The cell cannot continue with all of its energy carriers in this form so it has to reoxidise the carriers. In the case of yeast it does so by converting acetic acid to ethanol
CH3COOH + 4H+ + 4e- ➙ CH3 CH2 OH + H2O 2 ( e = electrons)
Acetic acid Hydrogen ➙ Ethyl alcohol water
The methaogens (a group of microbes that produce methane) produce methane by converting CO2 to METHANE and WATER
CO2 + 8H+ + 8e- ➙ CH4 + 2H2O
Carbon dioxide + Hydrogen ➙ Methane + Water
Each energy carrier transfers 2 electrons and 2 hydrogens, these equations show that converting CO2 to methane will reoxidise four carriers compared to reoxidising 2 if converting acetate to alcohol. Consequently conversion of CO2 to methane is a very efficient way of reoxidising the energy carriers.
Currently much research is directed at finding a bolus / vaccine that can be given to cattle to inhibit the pathway to methane.
One bolus that has been produced contains the chemical tribromomethane.
Methane ➙ TriBromoMethane
CH4 ➙ CHBr3
This is where 3 of methane’s hydrogen atoms have been replaced by Bromine atoms. The mode of action is called competitive inhibition. The tribromomethane gets into the the reaction site of the enzyme and blocks methane. 1080 and cyanide act in a similar manner.
In order to reoxidise the energy carriers, the microbes are forced to use another pathway or alternatively those microbes will give way to others who are not affected by the inhibitor. This will probably be far less efficient for the animal and cause slower growth and /or milk production.
On top of that there is the difficulty and danger in administering boluses every 100 days or so to the entire cattle stock of NZ. The expense alone coupled with the time involve could well make cattle farming uneconomic. On top of that, administering the bolus means putting a rod down the throat beyond the back of the tongue to get the bolus to a position where the cattle cannot spit it up. Cattle are powerful animals, this procedure is not without danger to the farmers - ACC beware. In addition, reaction from consumers overseas has been very strongly against cattle being given chemicals like this to reduce methane.
Conclusion. Production of methane is an important and natural pathway in metabolising dead matter. Blocking it will cause cells to find a less efficient alternative pathway.
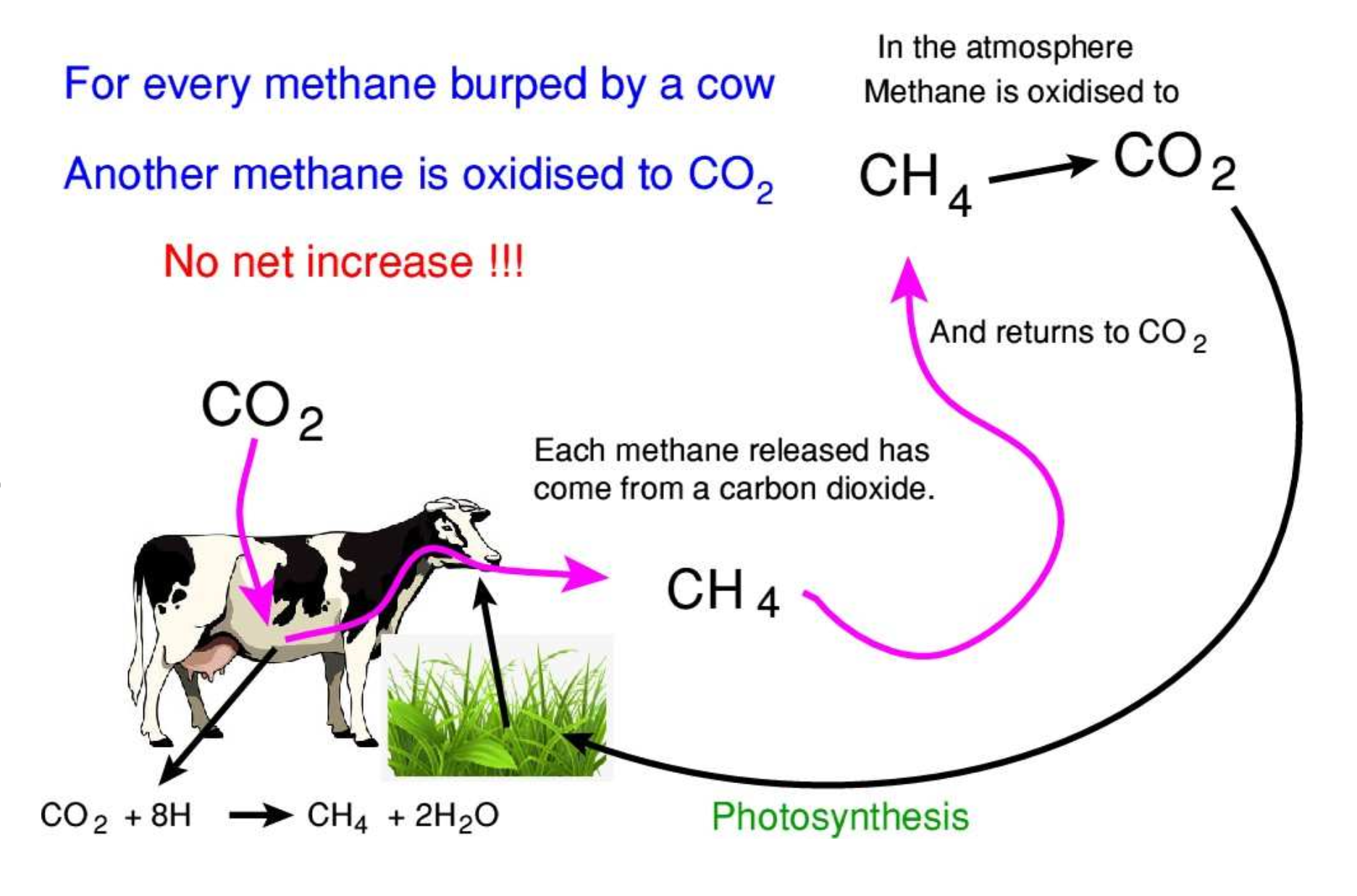
The Methane Cycle
As we have seen above, the methanogens take a CO2 from the air and convert it to methane and water. Once released into the atmosphere that methane comes under attack from hydroxyl radicals. These are charged particles that come from the natural splitting of water.
There are two reaction chains that can convert methane to CO2. Both are complex but one of them produces ozone and the other does not. This breakdown starts immediately the methane is released. The rate of breakdown increases with altitude with a significant proportion being oxidised in the stratosphere where it helps maintain a constant level of water vapour at about 4 ppm.
A Fatal Error
Much is made of the split gas approach whereby methane is deemed to last about 10 years in the atmosphere while CO2 is said (by the IPCC) to last for hundreds or even thousands of years.
This is nonsense, how long a molecule lasts in the atmosphere is of no consequence whatsoever.
The absorption and radiation of infrared in the atmosphere depends only on the concentration of the GHGs in the atmosphere today. If the level rises tonight then tomorrow will be a unmeasureable fraction warmer and if the level reduces tonight then tomorrow will be an unmeasureable tad cooler.
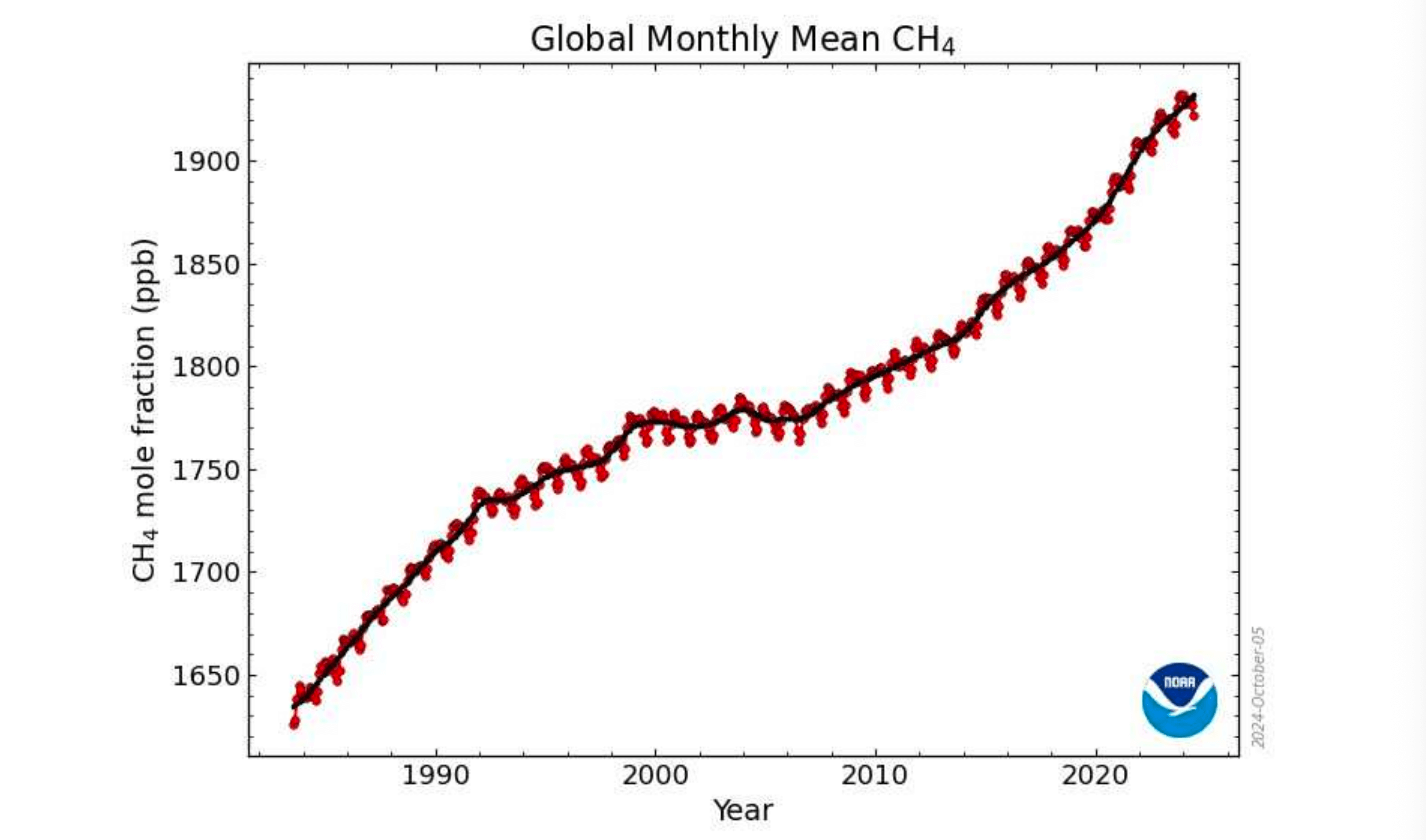
The graph of recent Global Monthly Mean CH4 shows that during the northern tropical rainy season CH4 rises by some 18 to 23.5 parts per billion (ppb) and during the dry season falls by 6 to 11.5 ppb.
Note also that the reduced slope of the graph post 2022 is due to increased oxidation.
Looking at the slightly bigger picture we can see that between 1998 and 2006 methane levels did not increase. This was also a time of global stagnation for temperature as well.
The clear message from these charts is that the level of methane in the atmosphere rises when production of methane exceeds oxidation and falls when production is less than oxidation. Global levels are therefore determined only by the relative rates of these processes
During that 1998 - 2006 period the methane released from all sources was equal to the atmospheric oxidation and therefore there was no net rise in the level of methane. That means that every methane released was merely replacing a methane that had been oxidised. This is where the IPCC methodology fails. The IPCC calculations of warming assume that every molecule released will add to the global level and will continue to affect warming for some mythical time period.
In reality, during that period there was no warming from added methane because it merely replaced methane that had been oxidised. It did not increase the global level of methane so it therefore had no warming effect at all.
Consequently, IPCC calculations of the warming effect of methane are wrong, totally wrong.
Change in the global level of CH4 = rate of formation (F) − rate of oxidation (O)
If F > O then the level rises. If F< O then the level falls. If F = O then the level stays constant.
How long a molecule lasts is of no consequence whatsoever.
Global Monthly Mean
.
This simple fact negates all IPCC calculations on the effects of Methane.
The same principle applies also to CO2.
Methane is a natural product of decomposition. It formation is fostered in warm wet conditions. The global increase in methane is thought to be related to increased global warmth.
Conclusion; methane concentrations are determined by the relative rates of formation and destruction. Longevity is of no consequence.
Sources Of Methane
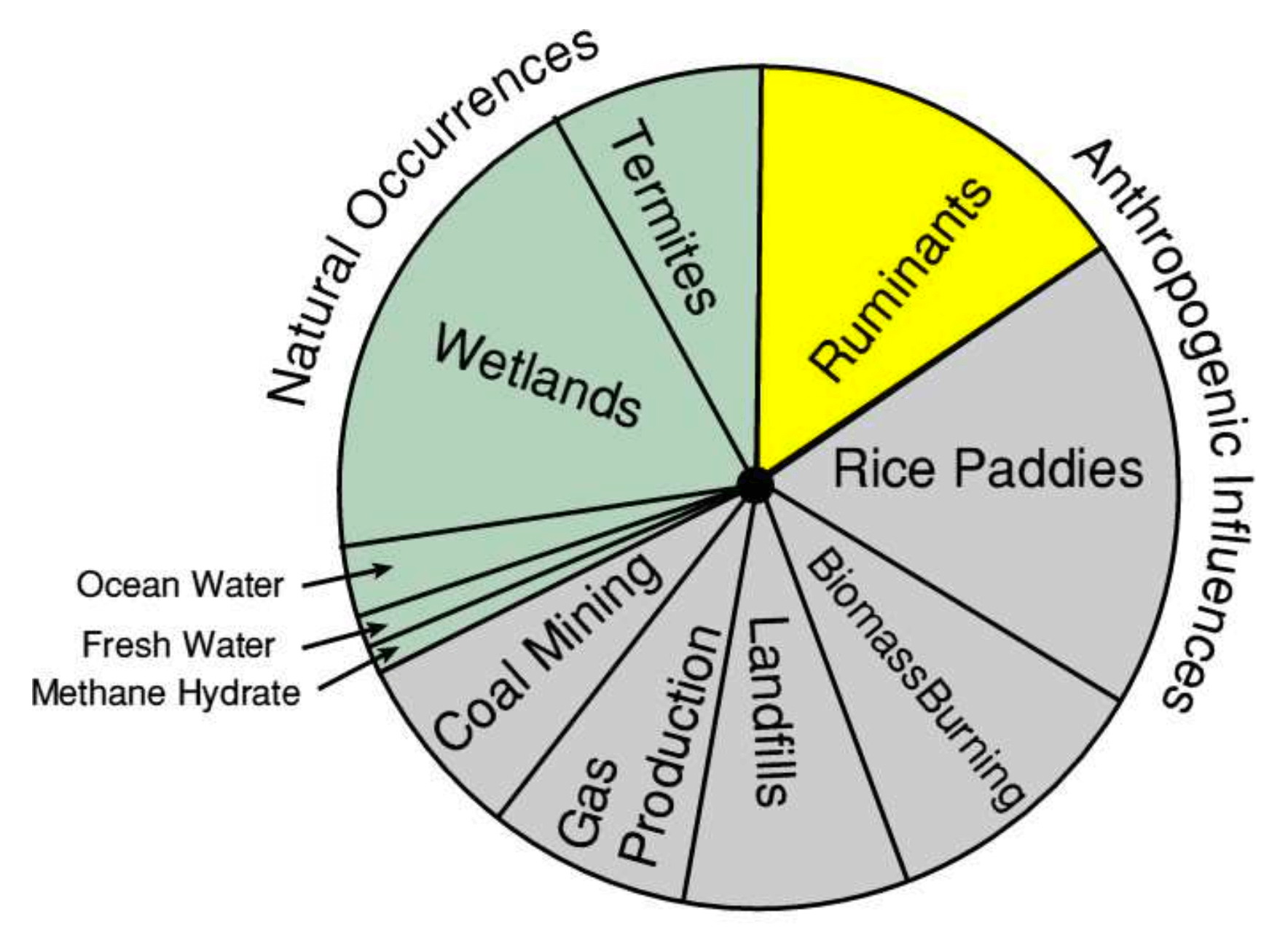
Before human settlements developed agriculture 10,000 years ago, the main sources of methane were; decomposition of dead matter and seepage from underground fossil fuel sources. The division in this chart into natural and anthropogenic raises questions of terminology.
METHANE SOURCES
Ruminants existed for millions of years before they were hunted by early Humans. Does the utilization of a natural resource make the animal anthropogenic? If we started to eat termites, would that make them anthropogenic? Rice plants existed long before Humans started cultivating them. Originally they were probably swamp plants, does our use of them make the source of methane anthropogenic ? Landfills merely concentrate human waste that in days of old would have been thrown out of sight to decompose, the end result is the same whether it is concentrated in a pit or decomposes somewhere else. Coal mining and gas production certainly increase the rate at which methane is released from fossil fuel deposits.
The proportion of methane that is classed as anthropogenic is therefore questionable.
Global Methane
Graphic at below shows concentration of methane as detected by the new methane satellite. Clearly, methane is a northern hemisphere issue in terms of atmospheric concentrations.
Note;
The significance of wetlands (green)
the proportion related to coal oil and gas in the Northern Hemisphere (red & blue)
that NZ does not feature that other anthropogenic is significant but not specified as to cause.
The high concentration methane in Arctic is due to several factors;
Permafrost thawing. Vast areas of land in the Arctic are permafrost which release methane when they thaw in the summer.
Methane Clathrates are mixtures of methane and water that form in a ratio about 6 water to 1 methane. Most clathrates are on the seafloor, trapped beneath arctic ice or in artic permafrost regions. When the ice melts or the water warms then methane is released.
Dead matter accumulates during the winters but is decomposed on warming during the summer.
How is global methane calculated.
These charts are from NOAA.
Methane Sources + Methane Sinks
I have already questioned the anthropogenic nature of the sources, put it this way, if humans ceased to exist, would the methane from animals (classed as livestock) or plants (Rice paddies), actually change ? There would still be ruminants roaming the world, the rice paddies would just revert to being wetlands so the emissions of methane would continue.
However, the value of these charts is the quantities of methane from the sources and sinks. The sources value is determined by taking the total methane in the atmosphere, and dividing it by the longevity of methane (this is very debatable value). The calculated amount of methane in the atmosphere is ~ 5285.5 million tonnes and at present increasing by ~ 21 million tonnes per year. On NOAA figures, that amounts to 97% of methane produced being oxidised and only 3% adding to the atmospheric concentration. Recent research suggests that the amount of methane released into the atmosphere is much greater than the above value and the rate of oxidation much higher. However in the absence of more accurate figures, the currently published values will be used.
When Beef & Lamb campaigned on getting farmers to “Know their Number”, the aim was that farmers should be aware of how much methane was produced by their stock and thereby give them a means of quantifying any reductions they were able to make. All for the purpose of reducing the tax that was to be imposed.
Their Number, then, was the total methane produced by their stock. What was ignored was that 97%+ of this Number, did not add to global methane, it merely replaced methane that had been oxidised to CO2. In other words, 97% of their Number, had no warming effect whatsoever, yet they were to be taxed for it.
In reality the situation is worse than that. Recent research shows that the uncertainties in methane released and in methane sinks is huge. For example, the authors of this paper by Bruce-Iri et al 2021 suggest that in grass pastures, the concentration of hydroxyl radicals is sufficiently high to oxidise all the methane produced by the cow. Grass, like all plants, release water vapour into the air as a consequence of having pores (stomata) open to the air in order to get CO2. This water, called evapotranspiration, is much higher over pasture than over non vegetated land. If the water vapour content of the air is much higher then so to is the amount of hydroxyl radicals that oxidise methane.
They postulate that grassy pastures may actually be methane sinks. However, the real value of this paper is the extent to which it points out how much we do not know about the sources and sinks of methane.
Reference : Bruce-Iri, Peter & Purnell, Max & Jehne, Walter & Arnold, Thorsten & Harris, Alfred. (2021). METHANE Sources, Sinks and Uncertainties. 10.13140/RG.2.2.28627.71201.
Conclusions;
Methane is a natural component of our atmosphere
Methane is mainly produced in the northern Hemisphere
NZ is irrelevant in terms of global methane
97+% of methane replaces methane that has been oxidized, it does not add to global concentration, it therefore has no effect on global temperature.
Continued in Part 2; Methane in New Zealand